Understanding pancreatic beta-cell death in type 1 diabetes through the use of systems biology
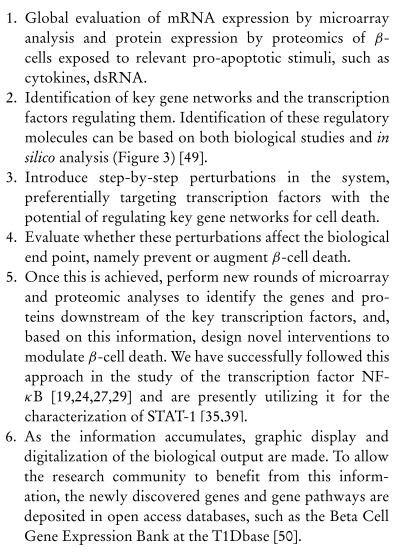
Pancreatic β-cells are the target of an autoimmune attack in T1DM. In addition to a progressive invasion of islets by mononuclear cells and an inflammatory reaction, known as insulitis, this causes a loss of 70-80% of β-cells at the time of diagnosis. The loss of β-cells occurs slowly and attained evidence suggests that increased apoptosis is the cause of this gradual β-cell depletion in T1DM patients. This gradual destruction of β-cells, being coupled with the autoimmune nature of the disease, proposes that T1DM is theoretically preventable. This initiative has commenced the foundation for several intervention trials. The two major intervention trials are individually based on the use of nicotinamide in the ENDIT trial, and insulin in the DPT-1 trial, unfortunately both have failed to prevent the onset of T1DM. There have also been attempts that have been made to replace the lost of β-cells by transplants of islets, which have been isolated from brain-dead organ donors. The initial 1 year results of islet transplantation, along with a steroid-free immunosuppressive regimen conveyed positive results. However, interest on this idea of transplant therapy has significantly decreased due to complications associated with the chronic immunosuppression and the gradual loss of islet function. In the end, this therapy resulted in less than 30% of its patients remaining free of insulin therapy after 2 years following the transplantations. Recent approaches that propose the restoration of β-cell function in T1DM patients, specifically the induction of β-cell regeneration along with inhibition of autoimmunity has still yet to be validated.
Our current knowledge of T1DM indicates that the disease has an identifiable pre-clinical phase that provides an opportunity to translate innovative therapies into disease prevention efforts, as well as reveling that islet transplantations has the potential to reverse the disease after clinical onset. In order to accomplish this there are a few questions that need to be answered,
“What should we do to successfully prevent T1DM or, once it is diagnosed, improve it by islet transplantation?” This question can be answered through inquiring two further questions,
(1) “Do we understand well enough how β-cells are progressively killed by the immune system in T1DM to allow a targeted intervention to prevent β-cell loss?”
(2) and “Is the current research approach to the problem, based on a narrow focus on individual pathways, adequate?”
Currently the present answers to both of these questions are no.
Up till now most research in this field of T1DM studies has greatly focused on the immune system, and has paid far less attention to the pancreatic β-cells. The main form of β-cell death in the course of insulitis is apoptosis, and it’s triggered by contact with activated macrophages and T-cells as well as the exposure to soluble mediators secreted by these cells, such as cytokines, oxygen free radicals and NO (nitric oxide).
“When β-cells are exposed in vitro to the cytokine IL-1β (interleukin 1β) or to IL-1β + IFN-γ (interferon γ), they present functional changes which are remindful of those observed in pre-diabetic patients, namely a preferential loss of the first-phase insulin release in response to glucose, probably caused by a decrease in the docking and fusion of insulin granules to the β-cell membranes, and a disproportionate increase in the proinsulin/insulin ratio.”
An exposure to IL-1β + IFN-γ or to TNFα (tumour necrosis factor α) + TNF-γ (but not to either cytokine alone), cause β-cells to undergo cell death mostly by apoptosis, with a minor necrotic component. Under these conditions, caspase 3 activation precedes the nuclear changes that are characteristic of apoptosis. IL-1β or TFN-α alone do not induce apoptosis in purified human or rodent β-cells, but when these cytokines are combined with IFN-γ, approximately 30-50% of the β-cells undergo apoptosis after 2-9 days. (Figure 1)
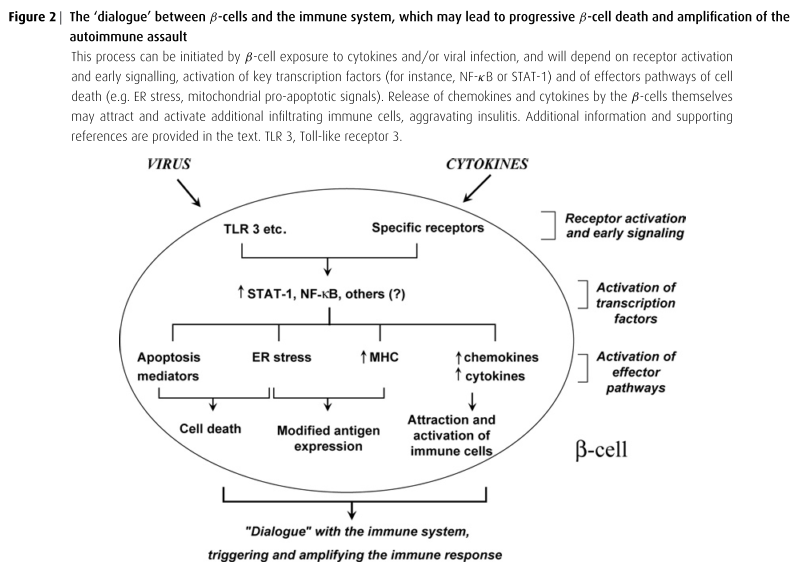
Cytokines promotes stress-response genes that either protect or contribute to the death of β-cells. They are also involved in the down-regulation of genes related to differentiated β-cell function and regeneration, along with triggering the expression of chemokines and cytokines that contribute to the attraction and activation of immune cells. This leads to a ‘dialogue’ between the target β-cells and the invading immune cells. There is an increased expression of MHC-related genes, which, when coupled to cytokine-induced endoplasmic reticulum stress, may modify antigen expression and augment recognition of the β-cells by the immune system. (Shown in Figure 2)
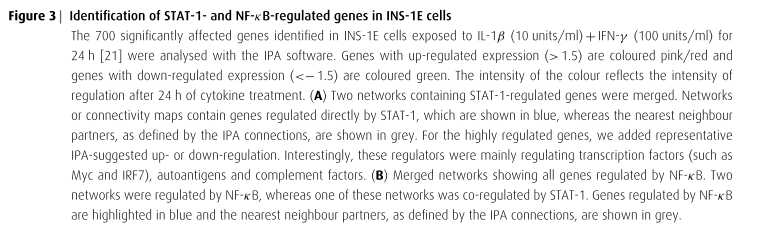
The extensive microarray experiments done within this study has identified nearly 700 genes that are up or down-regulated in purified rodent β-cells or insulin-producing INS-1E cells following 1-24 hours of exposure to the cytokines IL-1β and/or IFN-γ, along with nearly 2000 genes that were modified by cytokines or viral infection in the human pancreatic islets.
This study’s complete list of cytokine-modified rodent genes were entered into the Ingenuity Pathway Analysis (IPA) software which was used to identify networks and pathways that were regulated by cytokines. The top four pathways found that were significantly altered included those involving, antigen presentation, Nrf2 (nuclear factor-erythroid 2 p45 subunit-related factor 2)-mediated oxidative stress response, interferon signaling, and NF-κB (nuclear factor κB) signaling. In addition, a total of 28 networks that yielded P value scores greater than or equal to 10, as well as having more than 12 focus genes were identified. These networks identified key transcription factors and their interaction modes.
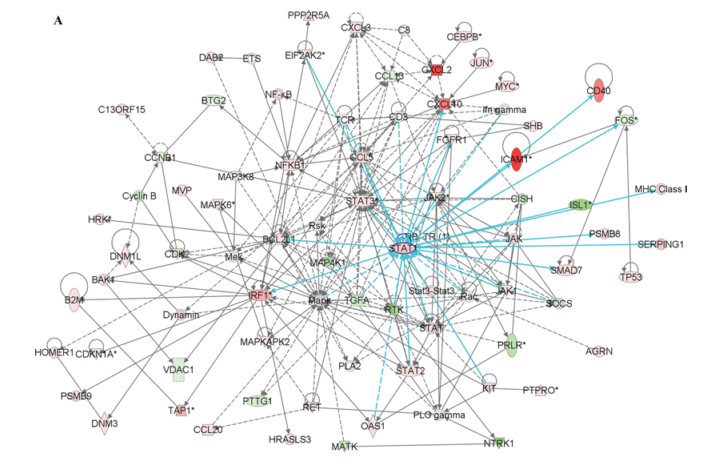
Two networks generated contained STAT-1, a signal transducer and activator of transcription, which is illustrated in figure 3A.
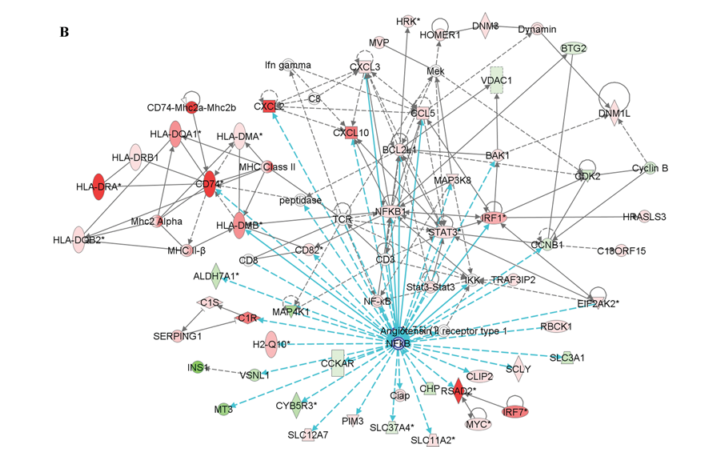
Two other networks constructed contained the transcription factor NF-κB, one of which also contained STAT-1. Figure 3B shows all the genes that are regulated by NF-κB.
The results of this study indicates that the fate of β-cells after an exposure to cytokines involves the perturbation of distinct β-cell gene networks regulated by NF-κB, STAT-1 and other transcription factors that have yet to be discovered. These outcomes suggest that the conflict leading to a progressive loss of β-cell in T1DM is definitely fought and to a great extent within the β-cells, which leads to a ‘dialogue’ between the invading cells from the immune system that may result in amplifying or dampening the immune attack.
Depending on the relative involvement of intracellular events within the β-cell or the immune system triggering diabetes in a specific animal model, intervening at the β-cell may or may not be sufficient while attempting to prevent the onset of the disease. From the observations made within this study, we can consider that the prevention of human T1DM will certainly require afflicting multiple targets, including, preventing the activation of pro-apoptotic β-cell gene networks, along with supporting β-cell defence/regeneration, as well as arresting/regulation of the autoimmune attack. This is an extremely difficult task to accomplish, and will require some novel approaches to solve these problem. The data also suggests that the contribution of the different pathways involved may also vary between individuals who are affected by T1DM, thus, indicating the need for an individualized therapy.
The fate of β-cells, following the exposure to immune mediators is an extremely complex and highly regulated process, depending on the duration and severity of distress put on the key interacting gene networks. Acquiring a full understanding of cellular responses during this transition from physiology to pathology requires a global multivariate strategy, as offered through a systems biology approach. The systems biology approach aims to develop models based on the comprehensive qualitative and quantitative analysis of the various components of a cell or tissue. The ultimate goal of this approach is to explain biological phenomena’s through the interaction of all its cellular and molecular elements.
At the end of this paper they offer a step-by-step approach, which is based on a systems biology method that will ultimately clarify the molecular mechanisms that are the source for β-cell death in T1DM patients. They believe that by following this strategy, it will be possible to fully map the interacting networks of the genes and proteins downstream from the pro-apoptotic signals which lead to β-cell death and amplification of the immune attack. Through attaining this knowledge, it will allow the search for a cure of T1DM to move from an experimental and blind approach to one that is genuinely mechanistically driven, resulting in the development of logical and targeted therapies to prevent and/or improve the disease.
This paper was extremely interesting to read. I don’t I have ever read anything quite like it, in regards to T1DM. Systems Biology has really struck an interest in me, after reading this paper along with the others in class. I plan to read more on this approach and its involvement with Type 1 Diabetes.
References
Eizirik DL, Moore F, Flamez D, Ortis F. Use of a systems biology approach to understand pancreatic β-cell death in Type 1 diabetes. Biochemical Society Transactions. 2008 Jan [cited];36(3): 321–327.